How Do Gas Particles Exert Pressure on Their Container
At standard pressure and temperature conditions most gases are taken. An ideal gas in simple words is a theoretical gas in which the gas particles move randomly and there is no interparticle interaction.

Give Reasons A Gas Exerts Pressure On The Walls Of The Container
When describing a container of gas the term pressure or absolute pressure refers to the average force per unit area that the gas exerts on the surface of the container.
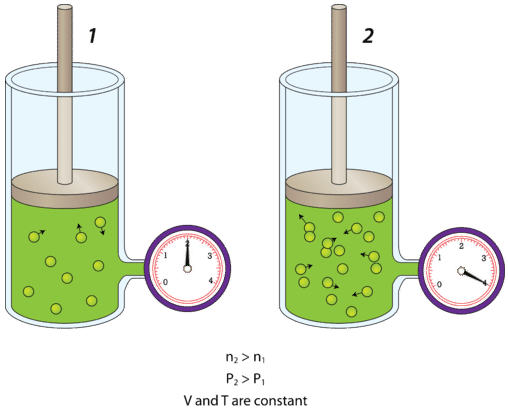
. Within this volume it is sometimes easier to visualize the gas particles moving in straight lines until they collide with the container see diagram at top of the article. The force imparted by a gas particle into the. An ideal gas doesnt exist in reality.
It follows the ideal gas equation which is a simplified equation we will learn further and is susceptible to analysis under statistical mechanics.

14 2 Factors Affecting Gas Pressure Chemistry Libretexts

Kinetic Theory Of Gas Pressure Video Kinetic Theory Gas Solid Liquid Gas

Kinetic Theory Collective Behaviour Of Large Systems Ppt Download Physics Lessons Kinetic Theory Physics
Comments
Post a Comment